Predicting Patient Responsiveness to rTMS Depression Treatment by Leveraging EEG Data.
This review looks at a recent study that uses EEG results to predict TRD patient responsiveness. It was found that responders have a consistently higher level of fronto-midline theta power than non-responders and that gamma-connectivity increases in responders...
Abstract
Though it is known that repetitive transcranial magnetic stimulation (rTMS) works for some patients with treatment resistant depression (TRD), a significant number of TRD patients do not respond to rTMS. Since it is resource-intensive for patients and clinics to conduct rTMS treatments, a predictive model based on accessible and non-invasively collected data for determining which patients will or will not react to rTMS would prove very useful. While predictors do exist, the data needed for them are often challenging to acquire or the models are not very accurate. This review looks at a recent study that uses EEG results to predict TRD patient responsiveness. It was found that responders have a consistently higher level of fronto-midline theta power than non-responders and that gamma-connectivity increases in responders while staying the same in non-responders as treatment continues. Using 30 features from EEG data across 39 participants, a predictive model was created and demonstrated a 91% ac-curacy for determining if a given patient will respond to rTMS treatment or not.
Introduction
According to a recent study conducted by the World Health Organization, over 250 million people worldwide suffer from depression (James et al, 2018). Though a variety of psychiatric and medical treatments exist for clinically depressed individuals, up to 20% of patients do not respond to standard treatments and have what is known as Treatment Resistant Depression (TRD) (Fava et al, 2003; Rush et al, 2006). This has led researchers to develop innovative ways to treat TRD patients, one of which is repetitive transcranial magnetic stimulation (rTMS). Though rTMS has been around for decades as a non-invasive treatment, its utility in depression treatment vs sham rTMS treatment is still regularly questioned (Amad et al, 2019), though supporting evidence is increasing (Kaster et al, 2019) and it is well accepted in the scientific community that the right kind of rTMS can improve the condition of TRD patients (Griffiths et al, 2019).
Despite the demonstrated effectiveness of rTMS, over 60% of TRD patients still do not respond to rTMS treatment (Berlim et al, 2014). Since rTMS treatment is costly and time consuming for both clinics and their patients (McClintock et al, 2018), the scientific and medical community would benefit significantly a predictive mechanism able to distinguish which patients could respond to rTMS treatment before beginning the 6-8 week treatment process.
There have been studies that used patient fMRI results as predictors for rTMS effectiveness (Drysdale et al, 2017), but these scans are also resource intensive on top of excluding patients who cannot have fMRIs performed. Another non-invasive method of collecting baseline patient data for predictive analysis is to conduct an electroencephalography scan (EEG). Only one other study by Arns et al, 2012 has made use of EEG results to predict patient reaction to rTMS treatment and found that 54% of non-responders could have been identified before beginning treatment, based on their baseline results. This study, however, only made use of one EEG scan before all testing began, where additional scans in the early stages of rTMS treatment could offer a more accurate prediction of patient responsiveness.
The current study aims to expand on these findings and improve the percent of non-responders identified by using data collected from each patient’s baseline along with a separate EEG scan after the beginning of rTMS treatment. Since the brains of patients responding to rTMS begin to show markers of change in cognitive function as a result of the treatment (Hoy et al, 2012), additional EEGs can be performed to add confidence to whether or not rTMS treatment would be useful for a given patient.
To do this, several noteworthy EEG outputs will be tracked for patients during the baseline test and follow up tests. Concretely, it is known that working memory (WM) activity is abnormally inhibited in depressed patients (Segrave et al, 2010) and that fronto-midline theta power and gamma power have been linked to WM performance (Jensen and Tesche, 2002; Herrmann and Mecklinger, 2001). Thus, both fronto-midline theta power and gamma power will be monitored for changes.
Furthermore, an increase in gamma-connectivity and increased connectivity between the dorsolateral prefrontal cortex (DLPFC) and other brain regions have been associated with response to rTMS treatment for depressed patients (Liston et al, 2014; Axmacher et al, 2010). By tracking all of the aforementioned markers, the present study aims to provide a model for classifying TRD patients into rTMS responding and rTMS non-responding groups to allow clinics and labs to better assign treatments to depressed patients.
Major Results
Fronto-Midline Theta Power
After titrated rTMS treatment was administered daily, five days a week, for three weeks, fronto-midline theta power was significantly larger in participants responding to rTMS than those not responding (p = 0.026). This result was stable for the baseline (BL) EEG, as well as the week one (W1) and final EEG.
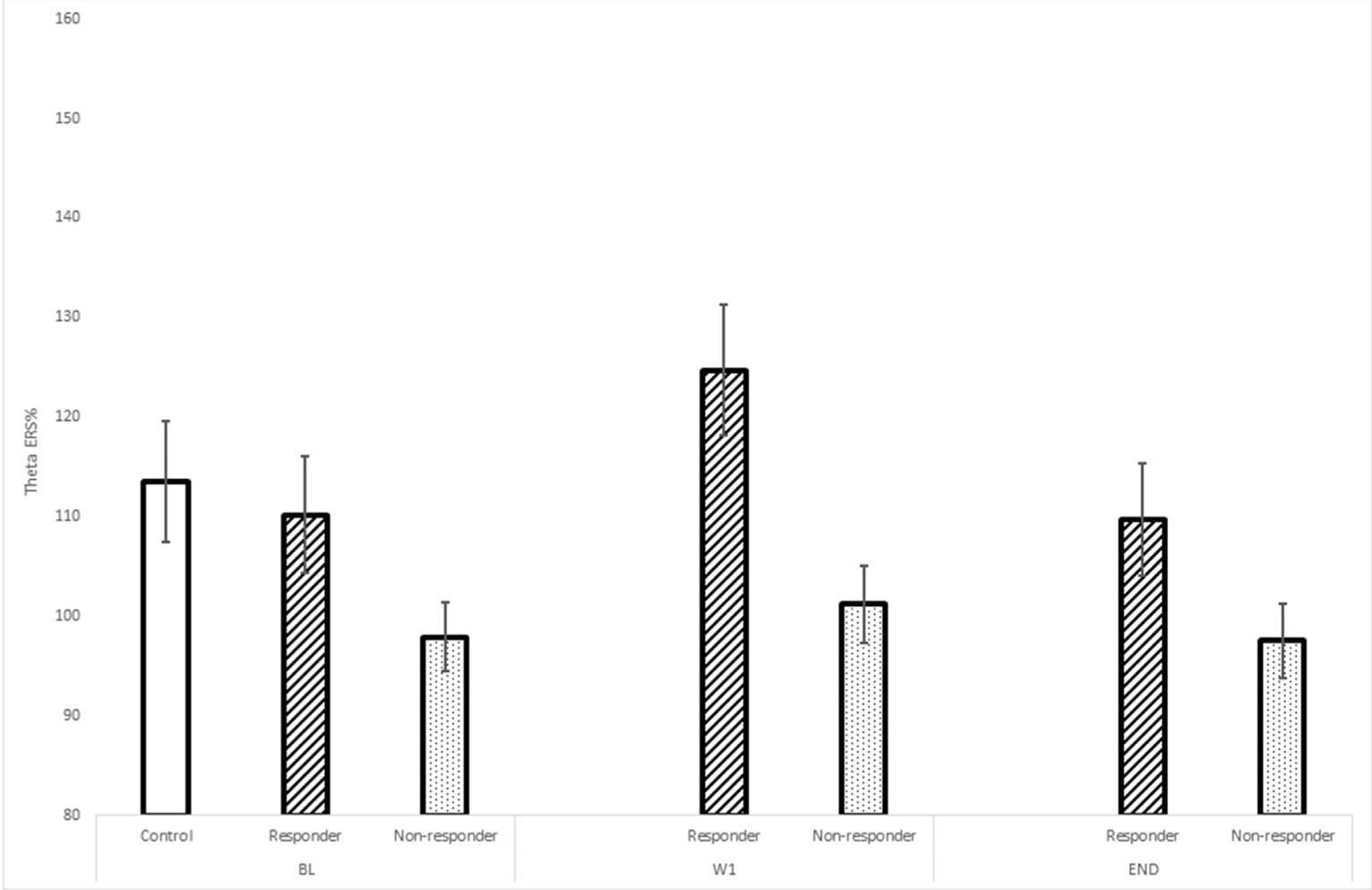
Connectivity
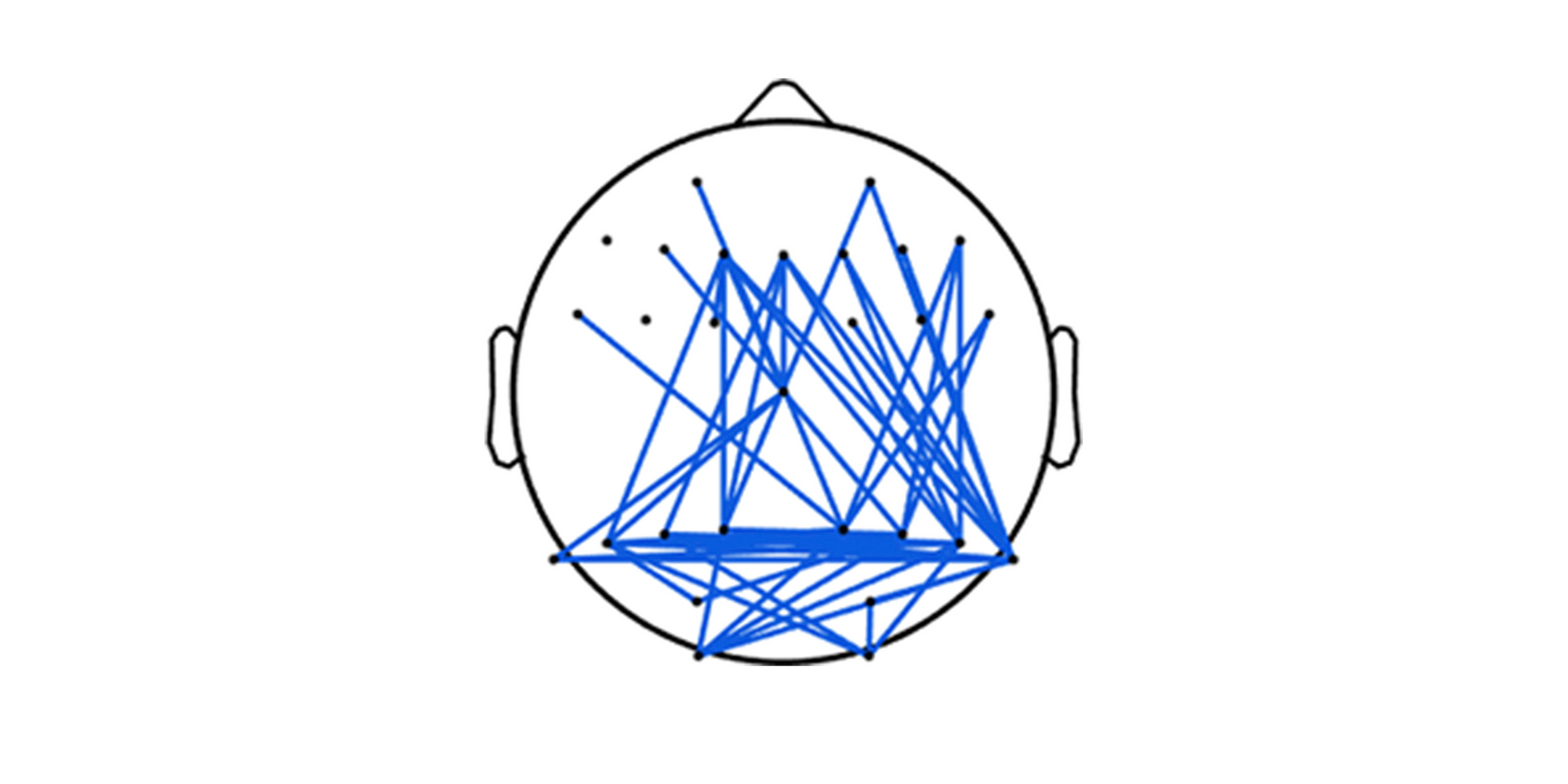
Similarly to theta power level between responders and non-responders, theta connectivity was significantly (p = 0.003) stronger in rTMS responding individuals in both the BL and W1 scans. Gamma connectivity, however, improved significantly (p = 0.001) for responders to rTMS treatment between BL and W1. This improvement did not occur for non-responders.
Response Prediction
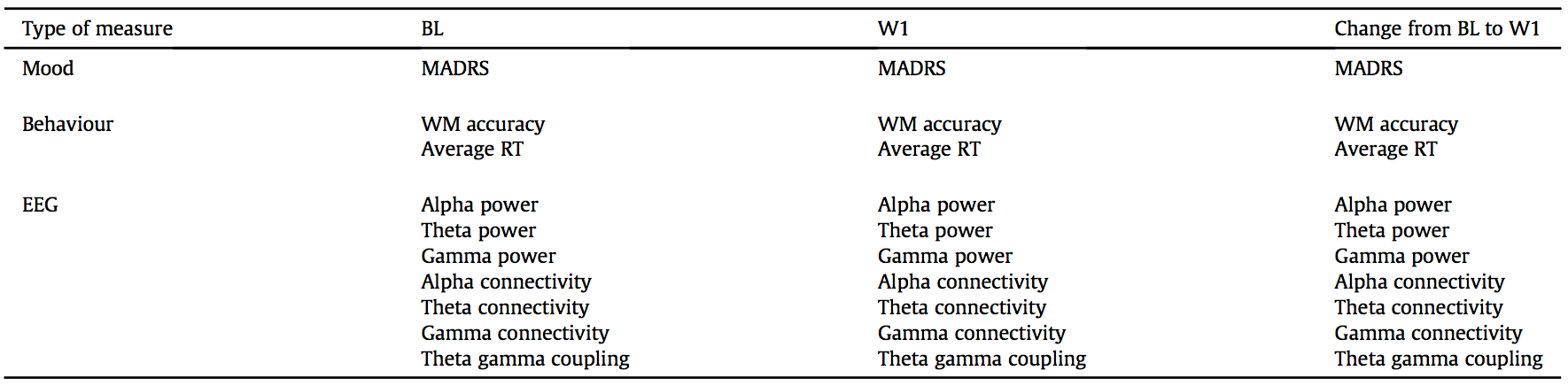
30 datapoints listed in Table 1 were used for all responders vs non-responders to rTMS treatment for depression to create a machine-learning model to assist in prediction of whether or not a given participant would respond to rTMS or not. The 30 features were used to train a linear support-vector machine (SVM) and by supplying the necessary data, the responsiveness of individuals could be predicted with a mean sensitivity of 0.9,1 specificity of 0.92, and sample accuracy of 91%.
Discussion
Though this study originally set out to find significant differences and changes in fronto-midline theta and gamma power along with theta-gamma coupling and increased connectivity, only gamma connectivity differentiated responders from non-responders by increasing from BL to W1 significantly in responders. Nonetheless, by way of demonstrating high accuracy with predictive modelling, the study demonstrated that there are indeed physiological markers that can be obtained non-invasively to predict if a patient with known TRD should receive rTMS treatment for depression.
Previous research by Arns et al. primarily made use of different physiological markers in their EEG study of TRD participants, but they did measure fronto-central theta power and found that it was larger in non-responders than in responders, in direct contrition with the findings of the current study. This result is presumably due to the different processes of recording theta power between Arns et al. and the current study. While this study measured theta power in WM-related tasks, Arns et al. measured theta power at rest. Support for this stems from the fact that theta power increases during both encoding and retrieval in WM tasks (Sauseng et al, 2004).
Also of note is the significantly increased gamma-connectivity in responsive participants versus non-responsive participants. Gamma-connectivity is linked to information retrieval and attention (Burgess and Ali, 2002), so the increase in gamma connectivity may indicate improving attentional ability in rTMS responding participants. However, there has been no conclusive research studying the effect of rTMS on gamma-connectivity and there was no significant change in Hamilton Depression Rating Scale (HAM-D) results, as would be expected with changes in gamma-connectivity, so this result yielded low confidence from this study’s researchers.
Critical Analysis
Though the need for a good predictive model for the effectiveness of rTMS on TRD patients is significant and a 91% accuracy is impressive, aspects of the sample used, the data collected, and the generalizability of the provided model lack much-needed robustness.
The most immediate issue of which to take note is the small sample size. With only 10 responders and 29 non-responders with usable data on top of the fact that the responder group had a larger proportion of males than the non-responder group along with further small sample-size issues, the generalizability the findings in the current paper are questionable.
Some results such as the theta power differences between responders and non-responders are firmly in line with past research and this lends credence to the other findings of this study. However, as mentioned above, the significance of the gamma-connectivity finding should be prioritized as something to validate in future research.
The last critique involves the claims of the authors about the robust nature of their SVM model for response prediction. With a sample size so small and the size of the responding vs non-responding group so disproportionate, the non-responders had to be weighed differently than the responders for the model to work. The claim that the model is not prone to overfitting is not necessarily true given the very small sample size and the comparatively large number of features, giving the model many degrees of freedom with which to construct its decision boundary. It is also unclear why the authors chose to use an linear SVM model over other small-dataset models such as a Radial Basis Function (RBF) SVM, Logistic Regression, or Naïve Bayes.
Future Directions
Future experiments should first and foremost consider an expanded and more generalizable sample of participants. All of the participants in the current study were recruited for the rTMS trial the Monash Alfred Psychiatry Research Centre in Melbourne, Australia. Further studies should aim, not only to obtain a larger number of participants, but also to randomize participant selection with a focus on a significant amount of non-WEIRD participants. If this study is replicated, results can be aggregated to see if the conclusions still hold.
Future studies should also ensure to repeat the gamma-connectivity increase seen in responders to rTMS between the BL and W1. As previously mentioned in this review, it is unclear why rTMS increased the gamma-connectivity metric and the HAM-D results don’t directly support this outcome either. Thus, a repeat with a different sample would help solidify the accuracy of this result. If gamma-connectivity increase is indeed expected of rTMS treatment from responsive patients, then research looking into the causal reasoning behind this event would be useful to the scientific community. Since gamma-connectivity is known to be related to attention (Burgess and Ali, 2002), the results of a simple attention test such as a Trail Making Test (Giovagnoli et al, 1996) at each testing interval (BL, W1, END) would further link increased gamma-connectivity with increasing attentional abilities for responsive participants. If research shows that gamma-connectivity does not actually improve in responsive patients, then it is critical to rework the linear SVM model without the additional parameter and test performance then.
Finally, future studies should compile all relevant data from both their studies and the current study and test several predictive modelling algorithms as opposed to one linear SVM model. As mentioned before, RBF SVM, Logistic Regression, and Naive Bayes, among others are options for this. Since prediction modelling for responsiveness to rTMS for participants with TRD is a very novel methodology and it is not yet clear exactly which EEG parameters should be included in the model as features, a computationally-intensive but important, task would be to run leave-one-out cross-validation (LOOCV) on the feature set to isolate which features the algorithm thinks are the most useful, from a prediction point of view. The output of this can demonstrate to researchers a potential avenue for future investigation for which metrics to focus on.
Author’s note: This research review was conducted for a neuroscience class during my undergraduate work at the University of Toronto. There are no conflicts of interest and this work has not been formally published. I found the idea of rTMS as a treatment mechanism quite interesting, especially when it yields novel (and working!) treatments for individuals with very serious TRD. I hope you enjoyed the work, if you have any questions about anything you read or feel that something needs clarification, please feel free to reach me at alex@alexgordienko.com or @alexgordienko_.
You can find the full formatted PDF of this review here.
References
Amad, A., Naudet, F., & Fovet, T. (2019). Repetitive transcranial magnetic stimulation for depression: The non-inferiority extrapolation. Journal of Affective Disorders, 254, 124–126. https://doi.org/https://doi.org/10.1016/j.jad.2019.01.006
Arns, M., Drinkenburg, W. H., Fitzgerald, P. B., & Kenemans, J. L. (2012). Neurophysiological predictors of non-response to rTMS in depression. Brain Stimulation, 5(4), 569–576. https://doi.org/10.1016/j.brs.2011.12.003
Axmacher, N., Henseler, M. M., Jensen, O., Weinreich, I., Elger, C. E., & Fell, J. (2010). Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 107(7), 3228–3233. https://doi.org/10.1073/pnas.0911531107
Bailey, N. W., Hoy, K. E., Rogasch, N. C., Thomson, R. H., McQueen, S., Elliot, D., … Fitzgerald, P. B. (2018). Responders to rTMS for depression show increased fronto-midline theta and theta connectivity compared to non-responders. Brain Stimulation, 11(1), 190–203. https://doi.org/10.1016/J.BRS.2017.10.015
Berlim, M. T., Van Den Eynde, F., Tovar-Perdomo, S., & Daskalakis, Z. J. (2014). Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: A systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychological Medicine, 44(2), 225–239. https://doi.org/10.1017/S0033291713000512
Burgess, A. P., & Ali, L. (2002). Functional connectivity of gamma EEG activity is modulated at low frequency during conscious recollection. International Journal of Psychophysiology, 46(2), 91–100. https://doi.org/10.1016/S0167-8760(02)00108-3
Carpenter, L. L., Janicak, P. G., Aaronson, S. T., Boyadjis, T., Brock, D. G., Cook, I. A., … Demitrack, M. A. (2012). Transcranial magnetic stimulation (TMS) for major depression: A multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depression and Anxiety, 29(7), 587–596. https://doi.org/10.1002/da.21969
Drysdale, A. T., Grosenick, L., Downar, J., Dunlop, K., Mansouri, F., Meng, Y., … Liston, C. (2017). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine, 23(1), 28–38. https://doi.org/10.1038/nm.4246
Fava, M. (2003). Diagnosis and definition of treatment-resistant depression. Biological Psychiatry, 53(8), 649–659. https://doi.org/10.1016/S0006-3223(03)00231-2
Giovagnoli, A. R., Del Pesce, M., Mascheroni, S., Simoncelli, M., Laiacona, M., & Capitani, E. (1996). Trail making test: normative values from 287 normal adult controls. The Italian Journal of Neurological Sciences, 17(4), 305–309. https://doi.org/10.1007/BF01997792
Griffiths, C., da Silva, K., De Vai, R., & O’Neill-Kerr, A. (2019). Repetitive Transcranial Magnetic Stimulation (rTMS) in Treatment Resistant Depression: Retrospective Data Analysis from Clinical Practice. Open Journal of Depression, 08(01), 16–28. https://doi.org/10.4236/ojd.2019.81003
Herrmann, C. S., & Mecklinger, A. (2001). Gamma activity in human EEG is related to highspeed memory comparisons during object selective attention. Visual Cognition, 8(3–5), 593–608. https://doi.org/10.1080/13506280143000142
Hoy, K. E., Segrave, R. A., Daskalakis, Z. J., & Fitzgerald, P. B. (2012). Investigating the relationship between cognitive change and antidepressant response following rTMS: A large scale retrospective study. Brain Stimulation, 5(4), 539–546. https://doi.org/10.1016/j.brs.2011.08.010
James, S. L., Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., … Murray, C. J. L. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392(10159), 1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7
Jensen, O., & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience, 15(8), 1395–1399. https://doi.org/10.1046/j.1460-9568.2002.01975.x
Kaster, T. S., Fitzgerald, P. B., Downar, J., Vila-Rodriguez, F., Daskalakis, Z. J., & Blumberger, D. M. (2019). Considerable evidence supports rTMS for treatment-resistant depression. Journal of Affective Disorders. https://doi.org/https://doi.org/10.1016/j.jad.2019.11.017
Knott, V., Mahoney, C., Kennedy, S., & Evans, K. (2001). EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Research - Neuroimaging, 106(2), 123–140. https://doi.org/10.1016/S0925-4927(00)00080-9
Liston, C., Chen, A. C., Zebley, B. D., Drysdale, A. T., Gordon, R., Leuchter, B., … Dubin, M. J. (2014). Default mode network mechanisms of transcranial magnetic stimulation in depression. Biological Psychiatry, 76(7), 517–526. https://doi.org/10.1016/j.biopsych.2014.01.023
McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., … Treatments, A. P. A. C. on R. T. F. on N. B. and. (2018). Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. The Journal of Clinical Psychiatry, 79(1), 16cs10905. https://doi.org/10.4088/JCP.16cs10905
Pfurtscheller, G. (2001). Functional brain imaging based on ERD/ERS. Vision Research, 41(10–11), 1257–1260. https://doi.org/10.1016/S0042-6989(00)00235-2
Pizzagalli, D. A. (2011). Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology, 36(1), 183–206. https://doi.org/10.1038/npp.2010.166
Rush, A. J., Kraemer, H. C., Sackeim, H. A., Fava, M., Trivedi, M. H., Frank, E., … Schatzberg, A. F. (2006). Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology, 31(9), 1841–1853. https://doi.org/10.1038/sj.npp.1301131
Sauseng, P., Klimesch, W., Doppelmayr, M., Hanslmayr, S., Schabus, M., & Gruber, W. R. (2004). Theta coupling in the human electroencephalogram during a working memory task. Neuroscience Letters, 354(2), 123–126. https://doi.org/10.1016/j.neulet.2003.10.002
Schutter, D. J. L. G. (2009). Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: A meta-analysis. Psychological Medicine, 39(1), 65–75. https://doi.org/10.1017/S0033291708003462
Segrave, R. A., Thomson, R. H., Cooper, N. R., Croft, R. J., Sheppard, D. M., & Fitzgerald, P. B. (2010). Upper alpha activity during working memory processing reflects abnormal inhibition in major depression. Journal of Affective Disorders, 127(1–3), 191–198. https://doi.org/10.1016/j.jad.2010.05.022